How To Find Vapor Pressure Of Ethanol

The Macroscopic View
The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); that is, the pressure of the vapor resulting from evaporation of a liquid (or solid) higher up a sample of the liquid (or solid) in a airtight container. Examples:
| substance | vapor pressure at 25oC |
| diethyl ether | 0.7 atm |
| bromine | 0.3 atm |
| ethyl booze | 0.08 atm |
| water | 0.03 atm |
The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The line on the graph shows the boiling temperature for water.
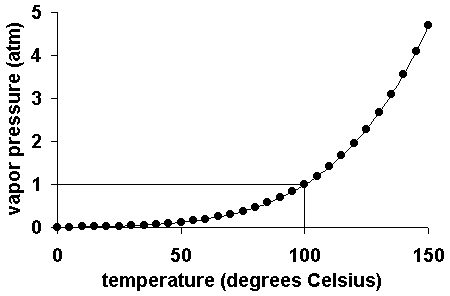
As the temperature of a liquid or solid increases its vapor pressure also increases. Conversely, vapor pressure decreases equally the temperature decreases.
The vapor pressure of a liquid can be measured in a diversity of ways. A simple measurement involves injecting a little of the liquid into a closed flask connected to a manometer. Click here for an illustration.
The Microscopic View
- When a solid or a liquid evaporates to a gas in a airtight container, the molecules cannot escape.
- Some of the gas molecules will somewhen strike the condensed phase and condense back into it.
- When the rate of condensation of the gas becomes equal to the rate of evaporation of the liquid or solid, the amount of gas, liquid and/or solid no longer changes.
- The gas in the container is in equilibrium with the liquid or solid.
- The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is chosen the vapor pressure level.
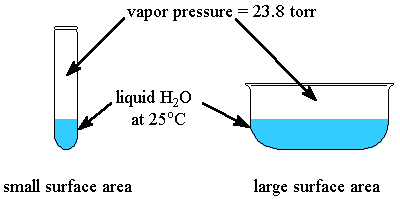
- Surface Area : the surface area of the solid or liquid in contact with the gas has no event on the vapor pressure.
- Types of Molecules : the types of molecules that brand up a solid or liquid determine its vapor pressure. If the intermolecular forces between molecules are:
- relatively potent, the vapor pressure level will exist relatively low.
- relatively weak, the vapor pressure volition exist relatively loftier.
- Temperature : at a higher temperature, more molecules have plenty energy to escape from the liquid or solid. At a lower temperature, fewer molecules have sufficient energy to escape from the liquid or solid.
| | ||
| ethyl ether (C4H10O) Pvapor (25oC) = 520 torr The relatively weak dipole-dipole forces and London dispersion forces betwixt molecules results in a much higher vapor pressure compared to ethyl alcohol. | ethyl booze (C2H6O) Pvapor (25oC) = 75 torr Although dipole-dipole forces and London dispersion forces also exist betwixt ethyl booze molecules, the strong hydrogen bonding interactions are responsible for the much lower vapor pressure compared to ethyl ether. |

Source: https://www.chem.purdue.edu/gchelp/liquids/vpress.html
Posted by: huynhhicum1949.blogspot.com


0 Response to "How To Find Vapor Pressure Of Ethanol"
Post a Comment